
I will definitely start with such a analogy which seems to give a quite simple, although thorough, description of how orbitals should be regarded. Takes into consideration the probability and region of This analogy describes an orbital fairly well, because it Sit in the lecture room, but one can say, with a high probability that this student will be within the confine of the OneĪlso usually cannot predict exactly where the student will However, oneĬannot be 100% sure that this student will be there because he or she may be absent from school on that day. In Lecture Room 1 at that time on that day. Weeks, there is a high probability that this student will be On Monday at 9.00 am, a student has to be in Lecture Roomġ for a chemistry lesson. An analogy forĪn orbital is the possible location of a student based on his Is a high probability of finding an electron. Why don't you try with analogies: at least, as a vary basic introduction, I very much appreciate the attempt done here by Goh et al.Īn orbital is defined as a region in space in which there
#Atomic orbitals explained free#
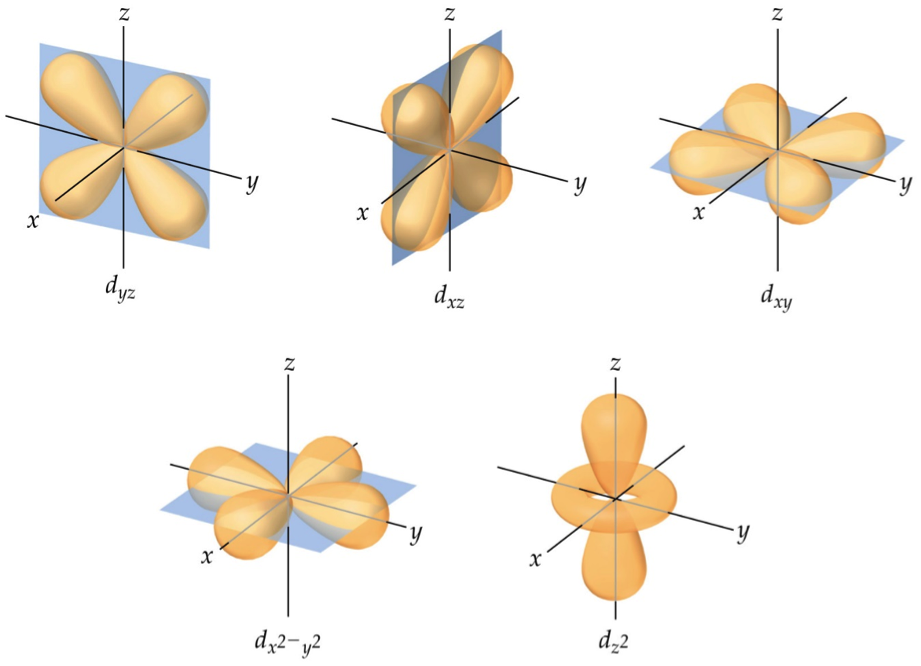
If you have any question, feel free to ask. Hopes this helps you understand orbitals. Since we generally take atomic orbitals to be the region of space that shows where the electron will be 95% of the time, the very outer sphere of the above image is said to be the orbital of the electron. Now if we superimpose all of these images, you will see something like this: Therefore an atomic orbital is a region of space that shows where the electron will be 95% of the time (usually we take 95% but it could be any number really such as 90% or 75%).Ī simple way of thinking of orbitals is that imagine that you have a magic camera that can take a sequence of photos of an electron in a hydrogen atom. Also known as atomic orbitals.Īn orbital is a well defined region of space. So how can we get around this problem? Well, if we can’t draw the orbit for an electron the next best thing we can do is to plot an electron density probability orbital for it. Therefore, it makes it impossible to plot an orbit for an electron as how can you? If you don’t know where the electron is going to be next, there is no way you can predict its path. So we actually can't construct an orbit and say that the electron is always going to follow that path. However the Bohr model assumes that you can precisely plot out where an electron is going to be, but we now know that due to the Heisenberg Uncertainty Principle you can’t precisely know the exact position and momentum of an electron. So basically what quantum theory tells us that we actually can't specify where and what a path an electron is going to take. I won't go into detail about it since to keep this explanation basic, but if you are interested check out this video which provides a really good explanation. This relationship is given by the Heisenberg Uncertainty Principle. So this basically means that we can't exactly know an electron's position and its momentum at the same time. It stated that electrons could not be treated as a classical particle and didn't not have a definite position and momentum. This is where quantum theory came to the rescue in the early 20th century. So how do electrons move around the nucleus? There are several issues with the notion that electrons travel in a circular orbit around the nucleus, the main one being that they should eventually undergo orbital decay and hence eventually slow down and crash into the nucleus. Despite this model being an excellent model at an introductory level, it doesn't tell the entire story.

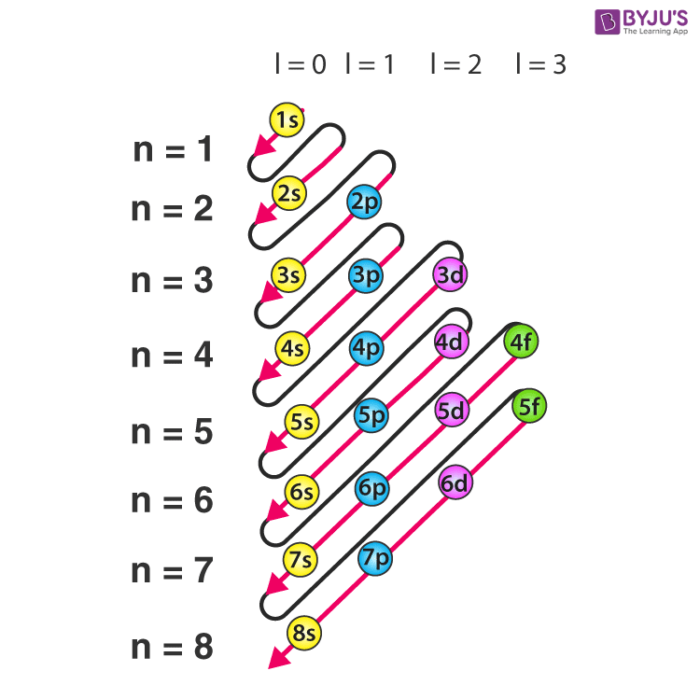
Well, the first step is to stop thinking electrons as being very small balls that orbit around the nucleus in a circular path.


 0 kommentar(er)
0 kommentar(er)
